
When you hear that ultraviolet (UV) light is a powerful disinfectant, it’s more than just “killing germs”. How UV really works is much more nuanced. It works at the molecular level, interfering with the fundamental macromolecules that allow living organisms to function. From DNA to proteins, UV light takes aim at the very building blocks of life and stops them in their tracks.
Let’s explore what’s really happening under the surface when UV disinfection is used, and get a deeper understanding of why it’s so effective against everything from bacteria and viruses to parasites, spores, and protozoan cysts.
UV disinfection is effective against nearly all types of biological contaminants. That includes:
But how is this one technology so broadly effective? It all comes down to the basic molecular machinery of all biological organisms: nucleic acids (DNA and RNA) and proteins. These biological macromolecules store genetic instructions, regulate cell activity, build cellular structures, and, importantly, allow pathogens to replicate, infect, and spread. And UV light, especially medium-pressure UV, is able to effectively disrupt all of them.
Not all UV is created equal.
That difference is key to why medium-pressure UV offers superior disinfection, especially in complex water systems where a variety of microorganisms and resistance mechanisms may be present.
When a photon of UV light strikes DNA, it absorbs that energy, and immediately tries to release it as heat. But during that infinitesimally brief moment while the energy is “trapped,” the DNA molecule becomes highly reactive.
This reactivity leads to a specific type of chemical event called a pericyclic reaction. In simple terms, two adjacent DNA bases (usually thymine or cytosine) fuse together into a structure called a dimer. Think of it like gluing two puzzle pieces together incorrectly: they’ll no longer fit where they should.
Why does that matter? DNA replication depends on precise base-pair matching. UV-induced dimers throw a wrench into that machinery. When the cell’s enzymes try to read or copy the DNA, they misread the fused bases, make mistakes, or grind to a halt. That disrupts all of the cell’s functions, especially reproduction.
Under the high-intensity lamps used in water disinfection, millions of dimers are formed at once. The cumulative effect is overwhelming to the cell, and it becomes inactivated – unable to reproduce or infect.
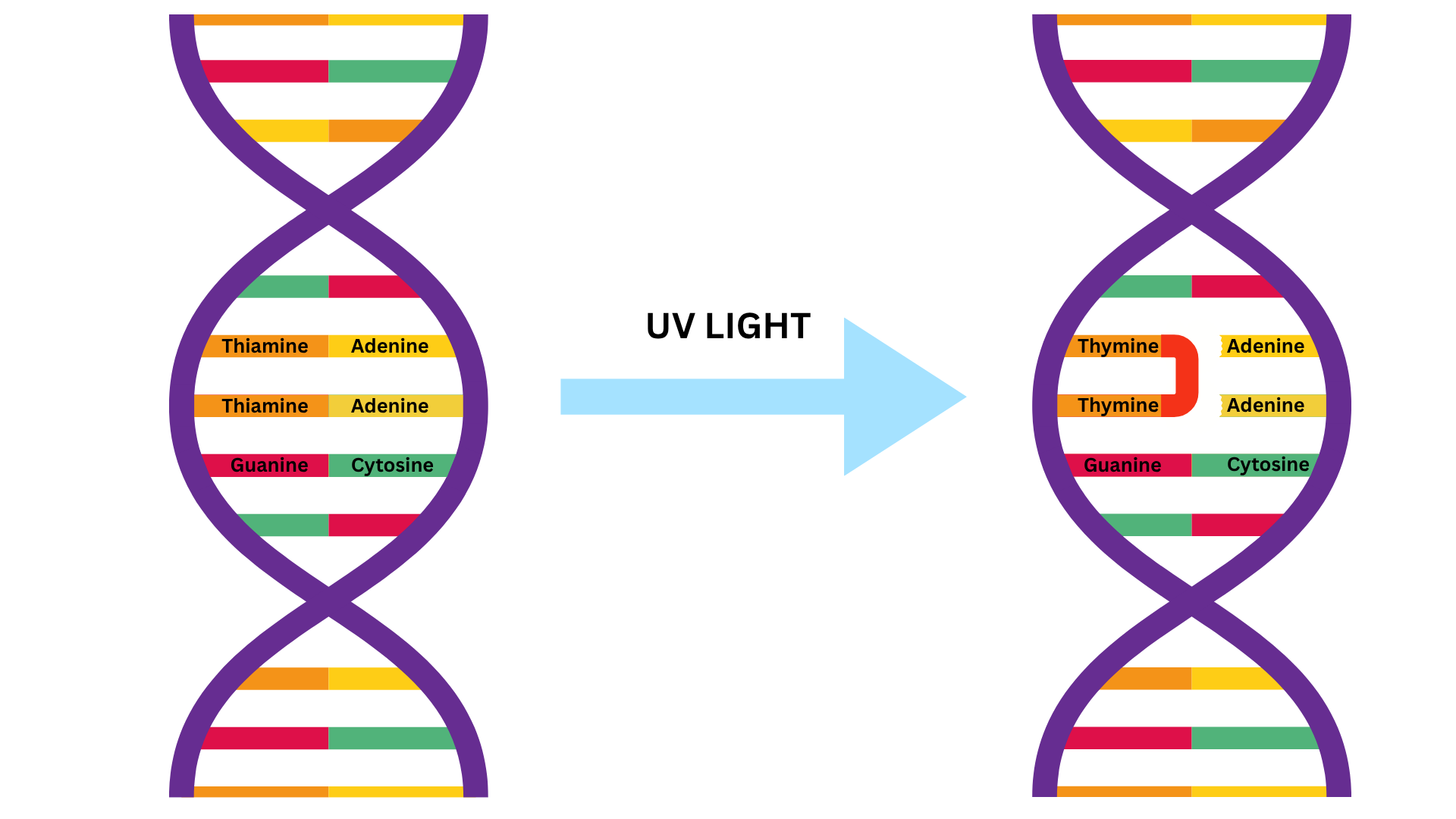
Important distinction: UV doesn’t “destroy” the cell immediately. The organism may remain physically present for a short time, but its ability to function is fundamentally compromised. In most cases, the cell will die shortly after being hit with a strong UV dose, but that effect is secondary to the UV-induced inactivation.
Many pathogens—especially viruses—rely on RNA, not DNA, to carry their genetic code. Fortunately, UV light can disrupt RNA too.
These lesions prevent RNA viruses from replicating or hijacking host cells, preventing infection.
Unlike low-pressure systems, medium-pressure UV includes shorter wavelengths—below 240 nm—which are especially damaging to proteins. Proteins absorb UV strongly in this range, and the consequences are significant:
This kind of damage complements DNA and RNA disruption, creating a multi-pronged attack that’s especially effective at high doses or against resistant microorganisms.
UV light can also cause indirect damage through the creation of reactive oxygen species (ROS).
Here’s how it works:
This condition is called oxidative stress, and it contributes to further degradation of biological molecules, compounding the amount of UV-induced damage. In simple terms, it’s a form of biochemical chaos that makes it almost impossible for the organism to recover.
Understanding these molecular mechanisms explains why UV is so effective as a point-of-entry (POE) solution for large buildings:
When UV is applied at the point where water enters a building, it acts as a biological firewall, stopping pathogens before they can circulate.
UV disinfection may look simple on the surface. But it’s more than a bright light in a tube, it’s a powerful tool rooted in foundational molecular biology. By damaging DNA, RNA, and proteins, and inducing oxidative stress, UV disinfection halts the essential life processes of microorganisms. They may still exist briefly, but they can no longer function or infect.
In short: UV doesn’t just clean water—it neutralizes biology itself.
As more buildings face water quality challenges from aging infrastructure, climate change, and microbial resistance, understanding the science behind UV disinfection helps us design smarter, more resilient, and safer water systems for the future.